Is there any benefit of adding Whole pelvic RT to Intermediate & high risk Ca prostate in comparison to Localized RT ?
Current recommendation still favoring whole pelvic RT despite at least 2 prospective randomized trials showing no benefit in 5 year PFS ?
Dear Abhinav
I am no expert on prostate cancer, but RTOG 94-13 has a very interesting story:
I believe this trial is one of the main reasons why WPRT is followed. It was first reported in 2003 and showed a benefit of WPRT in the whole group of enrolled patients:
Phase III trial comparing whole-pelvic versus prostate-only radiotherapy and
neoadjuvant versus adjuvant combined androgen suppression: Radiation Therapy
Oncology Group 9413.
Roach M 3rd, DeSilvio M, Lawton C, Uhl V, Machtay M, Seider MJ, Rotman M, Jones
C, Asbell SO, Valicenti RK, Han S, Thomas CR Jr, Shipley WS; Radiation Therapy
Oncology Group 9413. J Clin Oncol. 2003 May 15;21(10):1904-11.
PURPOSE: This trial tested the hypothesis that combined androgen suppression
(CAS) and whole-pelvic (WP) radiotherapy (RT) followed by a boost to the prostate
improves progression-free survival (PFS) by 10% compared with CAS and
prostate-only (PO) RT. This trial also tested the hypothesis that neoadjuvant and
concurrent hormonal therapy (NCHT) improves PFS compared with adjuvant hormonal
therapy (AHT) by 10%.
MATERIALS AND METHODS: Eligibility included localized prostate cancer with an
elevated prostate-specific antigen (PSA) < or = 100 ng/mL and an estimated risk
of lymph node (LN) involvement of 15%. Between April 1, 1995, and June 1, 1999,
1,323 patients were accrued. Patients were randomly assigned to WP + NCHT, PO +
NCHT, WP + AHT, or PO + AHT. Failure for PFS was defined as the first occurrence
of local, regional, or distant disease; PSA failure; or death for any cause.
RESULTS: With a median follow-up of 59.5 months, WP RT was associated with a
4-year PFS of 54% compared with 47% in patients treated with PO RT (P =.022).
Patients treated with NCHT experienced a 4-year PFS of 52% versus 49% for AHT (P
=.56). When comparing all four arms, there was a progression-free difference
among WP RT + NCHT, PO RT + NCHT, WP RT + AHT, and PO RT + AHT (60% v 44% v 49% v
50%, respectively; P =.008). No survival advantage has yet been seen.
CONCLUSION: WP RT + NCHT improves PFS compared with PO RT and NCHT or PO RT and
AHT, and compared with WP RT + AHT in patients with a risk of LN involvement of
15%. PMID: 12743142
And then… they did a subgroup analysis of only the neoadjuvant + concurrent hormonal therapy arm and again found a benefit for WPRT:
Whole-pelvis, "mini-pelvis," or prostate-only external beam radiotherapy after
neoadjuvant and concurrent hormonal therapy in patients treated in the Radiation
Therapy Oncology Group 9413 trial.
Roach M 3rd, DeSilvio M, Valicenti R, Grignon D, Asbell SO, Lawton C, Thomas CR
Jr, Shipley WU. Int J Radiat Oncol Biol Phys. 2006 Nov 1;66(3):647-53.
PURPOSE: The Radiation Therapy Oncology Group (RTOG) 9413 trial demonstrated a
better progression-free survival (PFS) with whole-pelvis (WP) radiotherapy (RT)
compared with prostate-only (PO) RT. This secondary analysis was undertaken to
determine whether "mini-pelvis" (MP; defined as > or = 10 x 11 cm but < 11 x 11
cm) RT resulted in progression-free survival (PFS) comparable to that of WP RT.
To avoid a timing bias, this analysis was limited to patients receiving
neoadjuvant and concurrent hormonal therapy (N&CHT) in Arms 1 and 2 of the study.
METHODS AND MATERIALS: Eligible patients had a risk of lymph node (LN)
involvement > 15%. Neoadjuvant and concurrent hormonal therapy (N&CHT) was
administered 2 months before and during RT for 4 months. From April 1, 1995, to
June 1, 1999, a group of 325 patients were randomized to WP RT + N&CHT and
another group of 324 patients were randomized to receive PO RT + N&CHT. Patients
randomized to PO RT were dichotomized by median field size (10 x 11 cm), with the
larger field considered an "MP" field and the smaller a PO field.
RESULTS: The median PFS was 5.2, 3.7, and 2.9 years for WP, MP, and PO fields,
respectively (p = 0.02). The 7-year PFS was 40%, 35%, and 27% for patients
treated to WP, MP, and PO fields, respectively. There was no association between
field size and late Grade 3+ genitourinary toxicity but late Grade 3+
gastrointestinal RT complications correlated with increasing field size.
CONCLUSIONS: This subset analysis demonstrates that RT field size has a major
impact on PFS, and the findings support comprehensive nodal treatment in patients
with a risk of LN involvement of > 15%. PMID: 17011443
Finally there was an update… but this is super complicated. You need the full text and you need to read the discussion carefully. It can be found on pubmedcentral. To summarize they found that WPRT still benefits people who get neoadjuvant + concurrent HT, but becomes detrimental in those who get short term adjuvant hormones. They feel that there is an interaction with timing of hormones and that could be a result of hormone related immune modulation. How cool is that!
An update of the phase III trial comparing whole pelvic to prostate only
radiotherapy and neoadjuvant to adjuvant total androgen suppression: updated
analysis of RTOG 94-13, with emphasis on unexpected hormone/radiation
interactions.
Lawton CA, DeSilvio M, Roach M 3rd, Uhl V, Kirsch R, Seider M, Rotman M, Jones C,
Asbell S, Valicenti R, Hahn S, Thomas CR Jr. Int J Radiat Oncol Biol Phys. 2007 Nov 1;69(3):646-55. Epub 2007 May 24.
PURPOSE: This trial was designed to test the hypothesis that total androgen
suppression and whole pelvic radiotherapy (WPRT) followed by a prostate boost
improves progression-free survival (PFS) by > or =10% compared with total
androgen suppression and prostate only RT (PORT). This trial was also designed to
test the hypothesis that neoadjuvant hormonal therapy (NHT) followed by
concurrent total androgen suppression and RT improves PFS compared with RT
followed by adjuvant hormonal therapy (AHT) by > or =10%.
METHODS AND MATERIALS: Patients eligible for the study included those with
clinically localized adenocarcinoma of the prostate and an elevated
prostate-specific antigen level of <100 ng/mL. Patients were stratified by T
stage, prostate-specific antigen level, and Gleason score and were required to
have an estimated risk of lymph node involvement of >15%.
RESULTS: The difference in overall survival for the four arms was statistically
significant (p = 0.027). However, no statistically significant differences were
found in PFS or overall survival between NHT vs. AHT and WPRT compared with PORT.
A trend towards a difference was found in PFS (p = 0.065) in favor of the WPRT +
NHT arm compared with the PORT + NHT and WPRT + AHT arms.
CONCLUSIONS: Unexpected interactions appear to exist between the timing of
hormonal therapy and radiation field size for this patient population. Four Phase
III trials have demonstrated better outcomes when NHT was combined with RT
compared with RT alone. The Radiation Therapy Oncology Group 9413 trial results
have demonstrated that when NHT is used in conjunction with RT, WPRT yields a
better PFS than does PORT. It also showed that when NHT + WPRT results in better
overall survival than does WPRT + short-term AHT. Additional studies are
warranted to determine whether the failure to demonstrate an advantage for NHT +
WPRT compared with PORT + AHT is chance or, more likely, reflects a previously
unrecognized biologic phenomenon. PMCID: PMC2917177 PMID: 17531401
This is a very interesting topic and I am sure you'll get loads of comments.
Indranil
Dear Indranil as you have mentioned RTOG 94-13 and i add another GETUG-01 trail …. both these are landmark trials that say that there is no PFS benefit of WPRT Vs Prostate only radiotherapy …. but still i think they maintain that standard of care should be WPRT and future trials should be based on findings of these trials. :
- Pommier P, Chabaud S, Lagrange JL, et al: Is there a role for pelvic
irradiation in localized prostate adenocarcinoma? Preliminary results of GETUG-
01. J Clin Oncol 25:5366-5373, 2007
However the interaction of Hormonal treatment with the effect of WPRT and PO RT are really interesting;
Hi
The standard for high risk is WPRT + hormones.
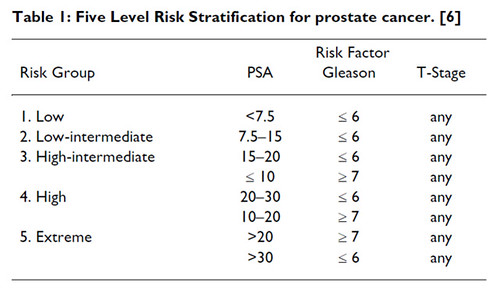
For intermediate risk it depends what situation you are dealing with. Soon you will see intermediate risk further classified in two groups.
Patients with low volume Gleason 7 (3+4), PSA <10 can be treated with Prostate RT Alone . It can be either Iodine Seed Brachy alone. Or a Combination of EBRT+ Seeds or EBRT + HDR brachy boost.
Where as Gleason 7 with PSA<20 with high volume of involvement will receive treatment more like high risk case.
Thanks for the papers guys.
Many Thanks Indraneel for the series of papers on RTOG 94-13 —it is interesting how the inferences have evolved over time with increasing followup duration .
The standard of care for high risk Ca Prostate remains WPRT+NHT till proved otherwise ,as the authors have concluded despite the controversies, while Nikhilesh has interestingly pointed out that soon the management of intermediate risk Ca Prostate will be divided into 2 broad categories based on Risk Stratification .
Interesting read . Thanks guys.
For all the brachy fans RTOG 0232 is one study to watch for Intermediate Risk Ca.
The other multi-centric ongoing clinical trials to watch are PROFIT and RADICALS.
Full paper available http://www.ncbi.nlm.nih.gov/pubmed/18423030